Ethical statement
This study was approved by the Institutional Animal Care and Use Committee of Seoul National University Hospital (IACUC; No. 20-0096-S1A2) and was performed in accordance with the Guide from our IACUC and the National Institute of Health Guide for the Care and Use of Laboratory Animals. All animals were maintained in a facility accredited by AAALAC International (#001169) in accordance with the Guide for the Care and Use of Laboratory Animals, 8th edition, NRC (2010). All methods are reported in accordance with ARRIVE guidelines.
Development of radiopaque biodegradable biliary stent using rotating rod-combined 3D printing system
PCL (MW = 43,000–50,000, PolyScience Inc., Warrington, PA, USA) was used as a biodegradable material. PCL is a bioresorbable polymer that has been used in FDA-approved medical devices, such as implants, drug delivery devices, and sutures. For radiopacity of the stent, a fine powder of barium sulfate (E-Z-HD; E-Z-EM Canada Inc., Quebec, Canada) was used. Barium powder was agitated uniformly in molten PCL on a hot plate (PCL with 25% [w/w] BaSO4, 110 °C).
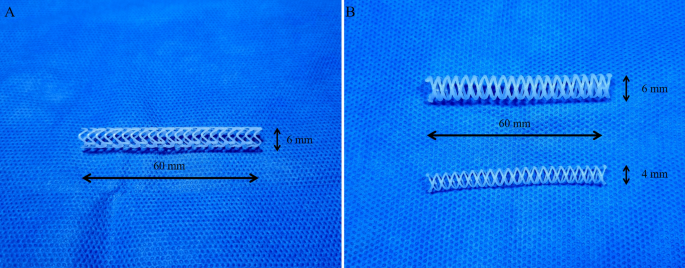
A rotating rod combined with a 3D printing system composed of a driving unit and extrusion unit was used to manufacture biodegradable stents16. The driving unit was operated by a G-code-based robot that consists of two heads moving translationally in the z-direction on the moving parts of x and y. The rotating rod used to print the stent was mounted on a rotating axis. The extrusion unit was composed of a heater and dispenser. The heater heated the material in the syringe to 250 °C. The molten material was extruded using a dispenser capable of providing pneumatic pressure from 0 to 700 kPa. The extrusion volume of the material could be modified using pneumatic pressure and nozzle size. The resolution of the 3D printing system is depending on printing temperature, extrusion pressure, and moving speed. It is possible to print the polymer from 150 μm to 1.5 mm. A rod was used as the substrate to print the tubular structure. Barium-loaded molten PCL was printed on a rotating rod to fabricate a stent with a strut of 500 µm. Our prototype PCL stent was 6 mm in diameter and 6 cm in length, and its shape was capable of loading into the delivery system used for percutaneous biliary intervention (Fig. 1A). However, the surgeon had a difficulty to insert prototype stent into the common bile duct (CBD) due to high flexibility of the stent. Therefore, we changed the design of the stent to make it easier to insert surgically. Considering the variation in CBD size depending on the pig, we made the final version PCL stent with a diameter of 4 mm or 6 mm and a length of 6 cm (Fig. 1B). The PDO (Resomer® X 206 S; CAS 29223-92-5, Sigma-Aldrich, St. Louis, MO, USA) stents for the radial force comparison test were fabricated in the same manner.
Stent radial force test
The radial force of the PDO and PCL stents during degradation in normal saline at 37 °C was examined. Radial force measurements of the two stents in each group (PDO and PCL) were performed after 5, 10, and 15 weeks. A compression test machine (Instron; Norwood, MA, USA) was used. The compressive velocity was 0.5 mm/s. The stent was fixed with a jig to prevent migration during measurement. Thereafter, the tubular-shaped probe compressed the middle of the stent in a cross-sectional direction to measure radial force.
In vivo animal experiment
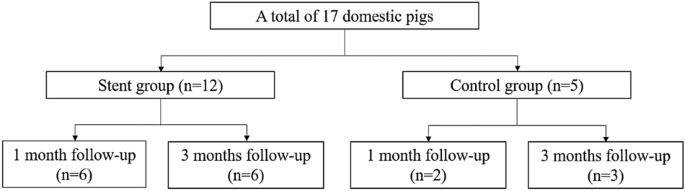
A total of 17 domestic male pigs (age, 3 months; weight, 26–30 kg) were divided into a stent group with 1-month follow-up (n = 6), stent group with a 3-month follow-up (n = 6), control group with a 1-month follow-up (n = 2), and control group with a 3-month follow-up (n = 3) (Fig. 2). 3 of the 6 stents used in stent group with a 3-month follow-up were prototype. All other stents were final version.
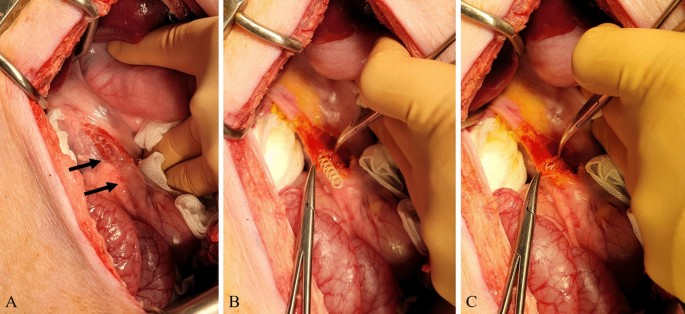
All surgical procedures were performed by two experienced hepatobiliary surgeons (Y.C. and E.S.H.). Anesthesia was induced using intramuscular injection of tiletamine-zolazepam (2 mg/kg; Zoletil 50, Virbac S.A., Carros, France) and xylazine (2 mg/kg; Rompun, Bayer HealthCare, Leverkusen, Germany). Thereafter, endotracheal intubation was performed by a veterinarian and general anesthesia was achieved using 1.5–2% isoflurane and oxygen. After thorough shaving and skin preparation, a sterile drape was applied to the area. A midline upper laparotomy was performed and the CBD was identified and exposed. The CBD was opened transversely (Fig. 3). In the control group, the CBD incision was closed with interrupted sutures using monofilaments (polydioxanone, PDS II 5-0, Ethicone, Somerville, NJ, USA). In the stent group, a 4-mm or 6-mm biodegradable biliary stent was inserted into the CBD according to the bile duct diameter (Fig. 3). When the CBD length was less than 6 cm, the stent was cut with scissors to fit the CBD. CBD incision-site closure was performed in the same manner as in the control group. The laparotomy was closed with multifilament and monofilament sutures in the peritoneum, fascia, and subcutaneous layers (polyglactin, Vicryl 0, Ethicone, Somerville, NJ, USA), and skin (nylon, Ethilon 3-0, Ethicone, Somerville, NJ, USA).
Animals were evaluated daily. Postoperative analgesia was administered via intramuscular injection of meloxicam (Metacam, Boehringer Ingelheim, Burlington, ON, Canada) at 0.4 mL/10 kg for 3 days. All pigs also received an intramuscular injection of cefazolin (Cefozolin, Yuhan, Seoul, Korea) at 20 mg/kg, twice per day for 3 days to prevent infection. Pigs underwent 1-month and 3-month follow-up CT examinations to evaluate the status of biodegradable biliary stents and the presence of postoperative complications, such as bile duct dilatation and biloma formation.
Finally, the pigs were euthanized 1 or 3 months after surgical implantation with intravenous injection of potassium chloride (2 mmol/kg) prior to intramuscular injection of 2 mg/kg tiletamine-zolazepam (Zoletil 50, Virbac S.A., Carros, France) and 2 mg/kg xylazine (Rompun, Bayer HealthCare, Leverkusen, Germany) for sedation. The entire CBD was sharply extirpated and immersed in 10% buffered formalin for histopathological investigation.
CT acquisition
CT examinations were performed using a commercially available, 64-channel CT scanner (Discovery CT 750 HD, GE Healthcare, Waukesha, WI, USA). Only non-contrast images were obtained to prevent unexpected adverse events in pigs following contrast injection. The CT parameters were as follows: detector configuration of 0.625 mm, tube voltage of 120 kVp, tube current of 150–200 mAs, and rotation time of 0.5 s. Axial images were reconstructed using a 2.5-mm slice thickness, and coronal multiplanar reconstruction (MPR) images were obtained using 3D imaging software.
Histopathology
CBD specimens were fixed for at least 72 h in 10% buffered formalin, processed following standard histologic procedure, and embedded in paraffin blocks. Hematoxylin and eosin (H&E) and Masson’s trichrome staining were performed on 4-µm thick sections. On H&E staining, the severity of tissue lesions suggestive of an inflammatory response of the bile duct, including (a) epithelial hyperplasia, (b) mucinous gland hyperplasia, (c) lymphoplasmacytic infiltration, and (d) neutrophil infiltration, was graded on a scale of 0–3 (Table 1)17. The items (a), (b), and (c) indicate chronic inflammation, while (d) reflects acute inflammation. The sum of these four item scores was calculated as the total tissue inflammation score for each specimen. On Masson’s trichrome staining, the degree of fibrosis was evaluated by measuring submucosal fibrosis thickness. The mean of three measurements in the most severely fibrotic area was used as the representative value for each specimen. All histological examinations were performed by an experienced pathologist (J.K.) blinded to the specimen group.
Statistical analysis
All results are reported as mean ± standard deviation. Differences in the tissue inflammation score and fibrosis thickness were compared using the Mann–Whitney U test. Statistical significance was set at P < 0.05. Statistical analyses were performed using MedCalc version 12 (MedCalc Software).