A Greeley hospital will serve as a hub for the distribution of COVID-19 vaccine, while city and Weld County health facilities are scheduled to receive more than 2,500 total doses in initial shipments, according to the Colorado Department of Public Health and Environment.
UCHealth Greeley Hospital, which is scheduled to receive 325 doses from the initial Pfizer allocation and 500 from Moderna, will assist the state health department in distributing vaccine to other locations.
The doses listed by the state are the doses to be administered by the Greeley hospital. The CDPHE does not indicate the number of doses the hospital will distribute.
According to the state health department, North Colorado Medical Center will receive 580 doses from Pfizer and 900 from Moderna in the first allocated doses. The Weld County Department of Public Health and Environment is slated to receive 200 doses of the Moderna vaccine.
The CDPHE on Friday released a list of 156 medical sites slated to receive the vaccine, and then distribute those to frontline health care workers as the first of the three-phase distribution plan begins in Colorado.
The state health department said it defined locations across the state with ultra-low temperature freezers to receive the first shipment of the Pfizer vaccine. The locations were chosen because of an ability to store, monitor and handle vaccines in temperatures from -60 to -80 degrees celsius, as well as a willingness to redistribute the vaccine to other providers.
The state also purchased 10 ultra-cold storage units, and CDPHE distributed eight of those units with the other two to be sent out on Friday.
On Friday evening, the U.S. Food and Drug Administration approved emergency use authorization for the Pfizer vaccine, which was shown to be 95% effective in preventing individuals from contracting COVID-19 based on large-scale trials.
Moderna, which also reported efficacy rates greater than 90% from its vaccine, followed Pfizer in applying to the FDA for emergency use authorization.
“With the vaccine arriving in Colorado soon, we are one step closer toward ending the crisis brought on by this once-in-a-century pandemic, but we must redouble our efforts to wear masks and avoid socializing these next few weeks,” Gov. Jared Polis said during a Friday news conference. “Our top priority has always been to save the maximum number of lives and to end this crisis as soon as possible.”
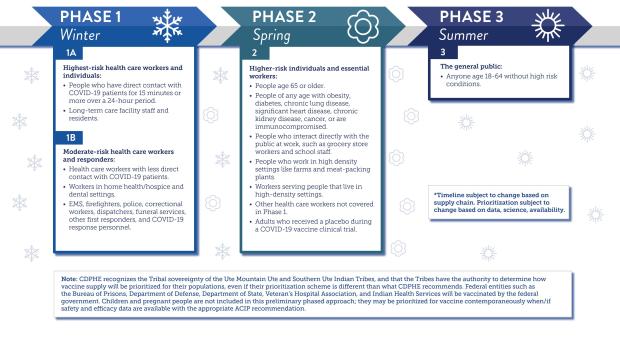
The state has a three-phase vaccination plan to inoculate Coloradans from a disease that has killed nearly 300,000 Americans according to data from Johns Hopkins University.
Governor Polis’ office said in an emailed release Friday that state health officials hope they get through vaccinations for Phase 1 this winter, and to the general population by the end of summer. The governor’s office added that timeline is dependent on steady shipments from the federal government and smooth administration by health care providers statewide.

UCHealth is involved with a third vaccine for COVID-19, as well as using an experimental drug called bamlanivimab that might lessen the severity of the disease in high-risk individuals. The FDA issued an emergency use authorization for the drug, which is administered on an outpatient basis for patients with mild to moderate symptoms.
More than 600 doses were sent to four UCHealth locations in Colorado. Poudre Valley Hospital in Fort Collins began using the drug on Dec. 1.
Banner Health is also using the drug, and doses were delivered Dec. 4 to patients at North Colorado Medical Center.
In a news release from Banner Health, Dr. Steven Loecke, chief medical officer for Banner in northern Colorado, said the drug offers patients more hope they will get through their illness.

UCHealth is a trial site for the vaccine developed by Oxford University and the pharmaceutical company AstraZeneca. The vaccine will be studied in approximately 30,000 people in the U.S. with a goal to enroll 1,500 of participants at UCHealth locations for the two-year study.
Dr. Gary Luckasen, the principal investigator of the trial and medical director of UCHealth’s clinical research program in northern Colorado, said late this week the health system is about halfway to meeting its goal of trial participants with 800 currently enrolled.
Luckasen said the AstraZeneca vaccine is easier to store than the versions from Pfizer and Moderna, but all three vaccinations appear to be safe and effective.
“In the next three to six to 12 months, a lot of vaccine will come through as people make them better and better,” Luckasen said. “All we know is they work well in the short term and seem to be safe. If we can get a foot up on the virus even in the short term, we’re doing positive things.”


