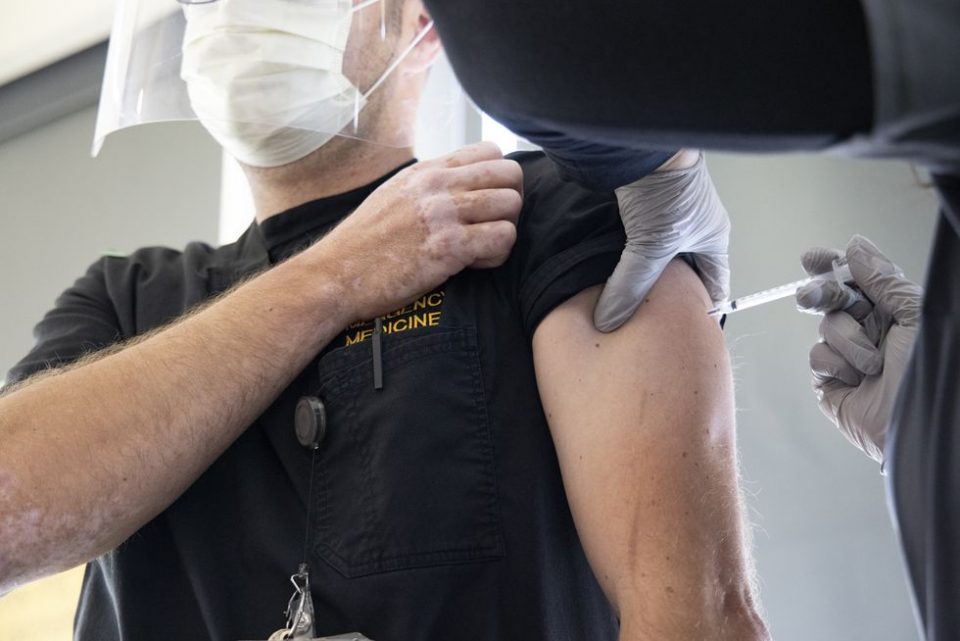
WASHINGTON (Gray DC) – The most-at-risk Americans and front-line workers are beginning to receive coronavirus vaccines Monday.
Last Friday, the F.D.A. gave ‘Emergency Use Authorization’ to a vaccine manufactured by Pfizer and its German partner BioNTech. Shipments began over the weekend. Some have already arrived and nearly three million doses are expected to be out for delivery by Wednesday.
HHS Sec. Alex Azar breaks down how they decided where to send the Pfizer vaccine
The government purchased, and drug makers produced, more than six million doses before the vaccine received clearance. But, because vaccination requires two doses with three weeks in-between injections, more than half of those pre-produced vials are being held in reserve.
The federal government is relying on FedEx and UPS to handle shipping. U.S. Department of Health and Human Services Secretary Alex Azar said delays associated with the holiday season should not impact timely delivery of the vaccine.
Meanwhile, Moderna’s promising vaccine candidate is poised to potentially receive its own Emergency Use Authorization Thursday or Friday. Azar said if all goes to plan, that drug could rollout along a similar timeline.
Sec. Azar on potential Moderna vaccine approval
If that’s the case, Azar said the country could send out 20-million doses of vaccine by the end of the month.
An estimated 24-million front-line medical workers and those in long-term care residents would be at the front of the line based on a plan endorsed by the Centers for Disease Control and Prevention. The C.D.C. estimates that 200-million Americans could qualify as high-risk and vaccination ahead of the general public.
Vaccines aren’t expected to have widespread availability until the Spring. Official estimates suggest health care workers will be able to vaccinate 30 and 50-million Americans in January and February respectively.
Over the weekend, President Donald Trump nixed plans to include himself and other White House staff in the first round of vaccination. The president was hospitalized in early October after contracting the virus. He has since suggested that he is now immune. But, Azar said there are still questions about whether prior infections offer immunity, and if so, how long it lasts.
Trial data on the vaccines suggest the vaccine they offer substantial protection for the vast majority of recipients. Test subjects who received the vaccination but still caught coronavirus had less severe symptoms. It’s unclear how long that protection lasts or whether it prevents the vaccinated from being infectious.
Data from the Pfizer and Moderna trials did not show risk of serious side effects associated with either drug. But, unforeseen complications are possible as the drug is given to a much larger number of Americans.
Azar said the administration will be monitoring for any ill effects and recently created a new smartphone app for vaccine recipients. The app will collect survey data and offer reminders when it’s time to receive a second dose.
Pfizer’s vaccine does pose a unique challenge. It needs to be stored at extremely cold temperatures and doesn’t last long once thawed. Azar said he’s confident the packaging designed by the drug makers are up to the task. Once at their final destination, he expects vaccines will be administered well before they expire.
Sec. Azar on Pfizer’s heat fragility
Copyright 2020 Gray DC. All rights reserved.