Ethical approval
The study was approved by the Helsinki University Hospital (HUS) Ethics Committee (Authorization number HUS/967/2017), and an institutional study permission was granted (§41/2017). All methods were performed in accordance with the relevant guidelines and regulations.
Tissue samples and histopathology
All tumor samples were re-evaluated for the following histopathological features: cell/stroma ratio, stromal composition (myxoid, chondroid, hyalinized, fat tissue), percentage of ductal structures, squamous metaplasia, mucous cells, sebaceous differentiation, oncocytic differentiation, mitotic features, and nuclear atypia. In addition, we analyzed the presence of tumor capsule, margin positivity, tumor diameter, tumor infiltration (budding) into the capsule, and multifocality. Single-author-analyses were first carried out (A.M.), and then experienced head and neck pathologists (A.L. and J.H.) re-evaluated the results.
Immunostainings
We collected all available tumor blocks from the study groups, based on their diagnostic slides. To prepare the tumor samples for immunohistochemical staining, we used the following protocol for both cyclin D1 and MIB-1: 4 µm thick slides were prepared from formalin-fixed paraffin-embedded (FFPE) tumor blocks, deparaffinized in xylene, and rehydrated with ethanol. Antigen retrieval was carried out by heat induced epitope retrieval (HIER), and the retrieval solution was pH 9, 15 min 98 °C. After that we deployed blocking of endogenous peroxidase and added primary antibody. For cyclin D1 we used the monoclonal rabbit anti-cyclin D1 antibody (SP4), ab 16663, Abcam with incubation time of O/N +5. For MIB-1, Dako Agilent monoclonal mouse antibody Ki-67/MIB-1 7240 (Dako Agilent, Santa Clara, USA) was used with incubation time of O/N +5. Secondary antibody (EnVision Flex, Dako, Clostrup, Denmark), chromogen (EnVision Flex DAB, Dako, Clostrup, Denmark) and substrate (Dako Mayer’s Hematoxylin, Dako, Clostrup, Denmark) were deployed. The staining process was performed with an Autostainer 480S (LabVision, UK).
Scoring
Immunopositivity in the tumor samples was scored by two investigators (A.M. and J.H.) who had no knowledge of the clinicopathological data. Nuclear and cytoplasmic expression of cyclin D1 were scored separately for each sample. Scoring of cyclin D1 expression was based on the percentage of nuclear and cytoplasmic immunopositivity in tumor cells. Scoring was as follows: negative (0), 0–10% expression; weak positivity (1), 11–40% expression; moderate positivity (2), 41–70% expression; and strong positivity (3), 71–100% expression. We used the following scale for MIB-1 expression: negative (0), 0–4% expression; weak positivity (1), 5–10% expression; moderate positivity (2), 11–20% expression; and strong positivity (3), > 20% expression. Breast tissue was used as a positive control for both cyclin D1 and MIB-1.
Patient selection and source of data
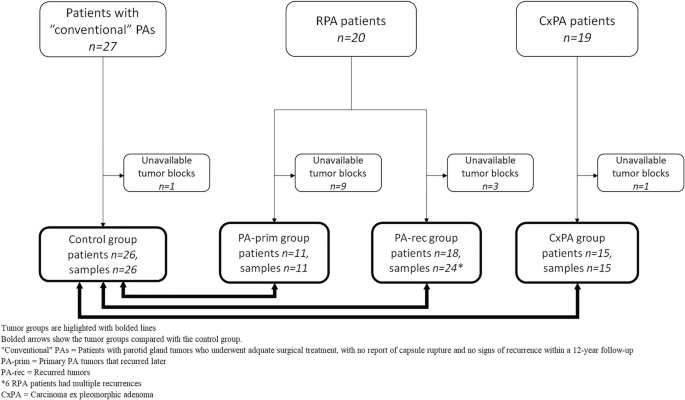
The electronic pathology archives and hospital patient records of the Helsinki University Hospital served as the source of data. We constituted three different main groups for comparison: “conventional” pleomorphic adenomas (PA) which served as the control group, recurrent pleomorphic adenomas (RPA), and carcinoma ex pleomorphic adenomas (CxPA). In the RPA and CxPA groups, we included patients who had a tumor in any major or minor salivary gland during the period 2000–2018.
The first group served as a control group and consisted of parotid gland PAs that had been treated adequately and showed no signs of recurrence within a 12-year follow-up. To achieve a long (minimum of 12 years) follow-up and assurance that the tumor had not recurred, we selected consecutive patients treated through 2005–2006. We included patients with parotid gland tumors who underwent adequate treatment, either superficial or partial parotidectomy, with no report of capsule rupture and who presented with a tumor ≥ 1.0 cm in diameter, to achieve enough material for histopathological analysis. We sent a questionnaire in a preaddressed, prepaid envelope to all patients to confirm their status at the time when data were retrieved, and to verify possible later contacts with any healthcare unit due to a salivary gland tumor. All 27 patients who fulfilled these criteria responded, and none reported health care visits or sequalae regarding the operated site.
In the RPA group, we included only recurrent tumors that appeared after an adequately treated primary tumor, i.e., those after superficial parotidectomy (for parotid gland tumors) and with no capsular rupture. Also, in this group we included only patients without evidence of a salivary gland malignancy during follow-up. We presumed that these selection criteria would best represent and identify tumors that are intrinsically prone to recur. Altogether 20 patients fulfilled these criteria and they experienced 27 recurrent events in total. Of these, six patients had a second recurrence, and one patient developed a third recurrence.
For histological and immunohistochemical studies, the tumors within the RPA group were split into two subgroups according to the sample investigated: primary PAs that were later known to recur (PA-prim) and recurrent tumors that appeared after the primary tumor (PA-rec). These subgroups were formed to study the clinical nature of the primary tumors in the PA-prim group compared to the control group more specifically, and to reveal potential histopathological changes in the tumor before it recurred.
In total, the PA-prim and the PA-rec groups contained tumors from 19 of the 20 RPA patients, because both the primary and the recurrent tumor blocks from one RPA patient were unavailable (Fig. 1). Tumor blocks were available from 11 PA-prim tumors from 20 patients. The cases with unavailable tissue samples had been treated elsewhere, and some of them decades ago. In the PA-rec group, 24 tumor blocks were available from 18 patients, some of whom had multiple recurrences. Tumor blocks were available from 15 out of the 19 CxPA cases, and from 26 out of the 27 cases in the control group. Table 1 further clarifies the division of RPA subgroups.
The CxPA group included 19 patients. Twelve of these patients were diagnosed de novo, whereas in seven patients, the malignancy occurred in RPA (one in the first recurrence, six in the second or later recurrence). None of the patients were included in both the CxPA and RPA groups.
Statistical analysis
The Mann–Whitney U test was used to compare scoring for expression of cyclin D1 and MIB-1 between the control group, RPA groups, and CxPA group as a pair comparison. The same test was used to compare the histological parameters with ordinal values. The Pearson chi-squared test was used to compare nominal values in suitable clinical and histological features. p value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS® software (version 27, IBM®).